What Are Cyp3a4 Inhibitors Used For

Review Article
Office of CYP3A4 in kinase inhibitor metabolism and assessment of CYP3A4 activity
Introduction
Tyrosine kinase inhibitors (TKIs) are pocket-sized molecules which arm-twist their furnishings on tumor (aberrant) cells by inbound these then inhibiting tyrosine kinase (enzymatic) functions. "The modest molecules frequently inhibit multiple enzymatic sites, have a wide spectrum of target kinases, and tend to be substrates of hepatic CYPs with a terminal elimination half-life of 12–24 hours, and thus require daily oral administration" (1). This judgement from Affiliate 62 of the "Goodman & Gilman'south" indirectly indicates that there might exist some issues apropos the right drug exposure. For most drugs, exposure [area under the plasma concentration fourth dimension bend (AUC)] is related to a drug receptor interaction which in turn elicits the pharmacological furnishings influencing the clinical outcome. The nearly important pharmacokinetic parameter determining drug exposure is drug clearance (drug emptying), because a given drug dose divided by the drug'south clearance results in drug exposure (AUC). The college the clearance, the lower the exposure. Whatever interindividual variability in drug clearance therefore results in variable drug exposure and this consequently modulates the pharmacological response.
CYP3A elimination
Most of today's available drugs are eliminated by members of the CYP3A isozyme subfamily (2). Between subject variability in drug clearance and hence exposure can be quite big and even more than variable, if drug-drug interactions interfere with a drug'due south clearance. CYP3A action can be modulated by co-medication over a 400-fold range either by inducing isozyme expression or inhibiting expressed enzymes (3). Therefore, dose adaptation seems to be the logical effect to ensure optimal drug exposure and thereby drug efficacy in each patient. For some drugs with narrow therapeutic index a therapeutic drug monitoring (TDM) to optimise drug efficacy or reduce drug toxicity is already established. Another approach, mainly used by researchers (4), is to appraise the action of CYP-enzymes with endogenous substrates or probe drugs similar midazolam or omeprazole. However, no routine testing of CYP3A activity has been established until today. In drug evolution all regulatory agencies recommend the thorough investigation of a compound'south potential to be a victim or a perpetrator of CYP (cytochrome P450) enzyme or transporter mediated drug-drug interactions (5,vi). However, in one case a new drug is bachelor on the market, most no measures are taken to individualise dosing. Instead, some combinations are contraindicated, but for other combinations where a dose adaptation could be used to reduce toxicity risk or enhance efficacy, a simple advice of "Practice caution with concomitant use of moderate CYP3A inhibitors" is given.
TKIs
Only recently an excellent and elaborate review on the pharmacokinetics of the TKIs has been published where the major factors responsible for the big interindividual variability in exposure of TKIs are discussed (7). Pocket-sized molecules like TKIs are administered orally which is a huge advantage for patients as they can accept the drug on a regular basis at home. Oral administration, still, can be associated with the risk of high beginning-pass metabolism resulting in much college doses to exist administered in order to ensure the required systemic exposure. This high starting time-laissez passer metabolism is mainly caused by CYP3A. To highlight the possible consequences, a theoretical example might explicate the underlying bug. A drug is administered orally and its absorption is assumed to be complete (100%). The fraction of the dose, which reaches the systemic circulation as parent drug is the bioavailability (F=20%). Hence, the first-laissez passer clearance eliminates 80% of the parent drug, which in this example is assumed to be mediated by CYP3A. Therefore, the individual bioavailability of this drug is inversely proportional to the CYP3A activity. It is therefore possible that bioavailability ranges from 5% to 50% between individuals, resulting in a 10-fold variability of exposure when the aforementioned dose is administered. This variability can be even higher if drug interactions occur, inducing or inhibiting CYP3A mediated metabolism. This example conspicuously shows that we demand to know the contribution of CYP3A to the overall clearance of the TKIs. An attempt has already been made (8) and by looking at Table 2, for all but one of the 15 TKIs listed a major contribution of CYP3A was declared, suggesting a stiff dependency of drug clearance from CYP3A. Nevertheless, further clearance mechanisms are not listed. In lodge to evaluate the importance and relevance of CYP3A for the clearance of the TKIs and hence their drug exposure to patients, all available European public assessment reports (EPAR) (9-20) of 31 TKIs (21-39) were evaluated regarding the clinical pharmacology data with special focus on clearance (6).
Mass residual of TKIs
As a showtime measure to evaluate clearance, the substances' route of elimination was assessed. Mass balance studies using radioactive labelled textile have been carried out for most of the TKIs and sampling of faeces and urine was performed. To evaluate the total drug clearance in relation to the CYP3A contribution, the first important parameter to know is the fraction of the dose captivated (fabs). Either this was listed in the EPARs, or information technology was calculated by adding the amount of drug excreted in urine (which must take been absorbed) to the proportion of metabolites analysed in faeces. However, when parent drug was detected in faeces, it could either be an unabsorbed fraction of the administered dose or an unchanged excreted fraction of the drug via bile into faeces after absorption. Consequently, there is some doubt nigh the faecal excretion of parent drug later on oral assistants. In fact, the calculated and listed fabs stand for the minimal absorbed fraction later oral assistants. Of the 31 TKIs, merely half are absorbed past at to the lowest degree l% of the orally administered dose (Figure ane). For dabrafenib, nintedanib, sunitinib, trametinib, and vandetanib, the EPARs do not provide any detailed data on the amounts of parent drug and metabolites excreted in faeces. Secondly, the absolute bioavailability is some other important parameter, which has been investigated for 21 of 31 TKIs (11 of them showing less than fifty% accented bioavailability). For substances, which are highly captivated, but show a low accented bioavailability, a substantial first-laissez passer metabolism can be anticipated. On the other hand, TKIs with a high bioavailability (>70%, dabrafinib, imatinib, regorafenib, ruxolitinib, and trametinib) are not expected to have a substantial contribution of CYP3A to their elimination in the first place, since less first-pass metabolism is observed. Apart from CYP3A being involved in first-pass metabolism, it also contributes to intrinsic clearance. If other pathways do not play an important role in drug elimination, fifty-fifty drugs with a loftier bioavailability can undergo CYP3A metabolism for drug elimination. All known bioavailability data are shown in Figure ii in relation to fabs. From the fraction absorbed and the absolute bioavailability information there is no prediction about the clearance mechanisms involved possible. Still, if the drug is highly absorbed, only has a low bioavailability due to a loftier commencement-pass metabolism, there is some likelihood that CYP3A is involved. The near likely candidate TKIs would be bosutinib and dasatinib (Figure 2) with fabs of at to the lowest degree 70% (dasatinib) and 55% (bosutinib) and a bioavailability of 34% (both).

Figure 1 The fraction absorbed (fabs) for the 31 TKIs, data extracted or calculated from EPAR documents. The light dark-green confined correspond the minimal fabs since no data on parent drug and metabolites excreted in faeces are given. TKIs, tyrosine kinase inhibitors; EPAR, European public assessment reports.
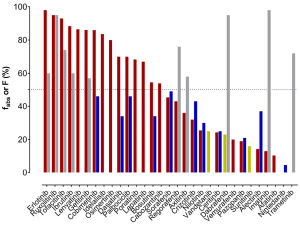
Figure 2 Absolute bioavailability of 21 TKIs, data extracted from the EPAR documents. Gray bars are used for F>50%, blue bars for F<fifty%. For comparing, fabs data are included for all 31 TKIs. TKIs, tyrosine kinase inhibitors; EPAR, European public assessment reports.
Equally an instance, a closer look at bosutinib and dasatinib is taken. Extensive in vitro studies take been carried out with bosutinib (15). CYP3A4 is the predominant enzyme capable of bosutinib metabolism whereas CYP1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, or 3A5 did not metabolise bosutinib. There is quite a poor description of the metabolism of dasatinib in the EPAR (21). Based on in vitro data, it was claimed that CYP3A4 appears to play a major office in dasatinib metabolism. However, dasatinib is also able to inhibit the activities of CYP2C8 (Chiliadi=3.6 µM) and CYP3A4 (Thousandi=1.9 µM), the latter one being time dependent. At concentration 10-fold higher than Cmax dasatinib did not induce CYP3A4, 1A2, 2B6, 2C9, and 3A4 in primary cultures of human hepatocytes. For both TKIs information are inconclusive regarding their clearance processes.
Drug-drug interaction studies
To gain farther inside into the office of CYP3A in the metabolism of TKIs, results of drug-drug interaction studies can be used. These studies are generally part of the drug development programme and the FDA clearly states (5): "The objective of drug-drug interaction studies is to decide whether potential interactions between the investigational drug and other drugs exist and, if so, whether the potential for such interactions indicates the need for dosage adjustments, additional therapeutic monitoring, a contraindication to concomitant use, or other measures to mitigate risk." In addition, the identification of the major routes of elimination, the relative contribution of enzymes and drug transporters to drug disposition should be carried out. If all in vitro studies have been performed to assess whether a drug is a substrate, inhibitor, or inducer of metabolising enzymes, studies with strong inhibitors and inducers should be carried out to provide a sensitive cess of the interaction potential. Therefore, if the contribution of CYP3A to the overall elimination of a drug accounts for ≥25% of the clearance, drug interaction studies with a strong CYP3A inhibitor (i.east., ketoconazole) and strong CYP3A inducer (i.east., rifampicin) must be carried out. Almost of the TKIs have been investigated using ketoconazole and rifampicin. As an alphabetize for the degree of inhibition the ratio of AUC with and without perpetrator (AUCR) is used. AUCR data from 17 TKIs with both ketoconazole and rifampicin are presented in Effigy three. The highest AUCR during ketoconazole was 25 for ibrutinib, the strongest consecration by rifampicin was observed for bosutinib (AUCR =0.06). To evaluate a possible human relationship between AUCR ketoconazole and AUCR rifampicin a nonlinear regression assay was performed with the log transformed data of the 17 TKIs bachelor. At that place was a significant linear regression betwixt both AUCRs (rtwo=0.6951) (Figure 4), which is not surprising since ketoconazole is a stiff inhibitor of CYP3A and rifampicin a strong inducer of CYP3A although other effects might be present additionally. Therefore, if CYP3A is a major contributor of a drugs clearance a high AUCR for ketoconazole and a depression AUCR for rifampicin is expected. Of course this relationship can be weakened by other mechanisms. Likewise being a strong inhibitor (AUCR ≥five) of CYP3A, ketoconazole is a weak inhibitor (AUCR ≥1.25 but <2) of CYP2C19, CYP2C8, and an inhibitor of the drug transporter P-gp (5). Rifampicin on the other hand is a stiff inducer (AUCR ≤0.2) of CYP3A, a moderate inducer (0.2< AUCR ≤0.v) of CYP2B6, CYP2C8, CYP2C9, CYP2C19. It induces the drug transporter P-gp, merely likewise inhibits OAPT1B1 and OATP1B3 (5).
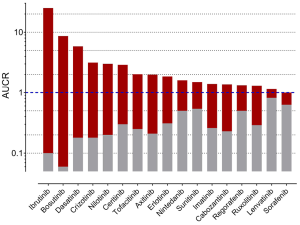
Figure 3 AUC ratio (AUCR) for all TKIs where interaction studies with ketoconazole (ruby-red) and rifampicin (gray) were carried out. AUCR =1, no interaction; AUCR >one, inhibition; AUCR <ane induction. TKIs, tyrosine kinase inhibitors.
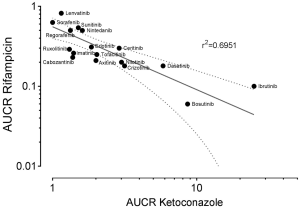
Figure 4 Human relationship between the log transformed AUC ratio (AUCR) of ketoconazole (>1) and rifampicin (<1). A to the lowest degree squares assay has been performed showing the resulting line with the 95% confidence intervals.
Concerning CYP3A involvement in TKI metabolism, there is conspicuously an overestimation in published literature, which might take been adopted by some clinicians. Sunitinib might serve as an example. Data extracted from the sunitinib EPAR (35) show that 16% of the dose is excreted via urine and 61% via faeces. The study referred to in the EPAR document reveals that 77% is excreted inside 3 weeks after sunitinib assistants; nevertheless that major portion was excreted during the starting time 7 days (40). Faecal excretion accounts for 48.2% with 25% of the sunitinib dose beingness the active metabolite SU012662, followed past the parent drug thirteen.6%. In urine, like amounts of sunitinib and SU012662 (half dozen.4% and 6.9%) are constitute. Improved survival and increased toxicity have been associated with increased exposure to sunitinib (41,42). Depending on the indication patients usually receive a fixed dose of either 37.5 or 50 mg sunitinib once daily. An boilerplate sunitinib clearance of near 600 mL/min has been reported by various sources (35,43), dose adapted trough concentration varied 8-fold for sunitinib and 18-fold for the active metabolite SU012662 (43). "Sunitinib is metabolised primarily by the cytochrome P450 enzyme, CYP3A4, to produce its primary active metabolite, which is farther metabolised by CYP3A4" which is a quotation of the prescribing information of Sutent (44). This has led to several studies evaluating the value of CYP3A activeness or the influence of pharmacogenetics on sunitinib exposure. A cohort of solid tumor patients (n=52) starting with sunitinib was phenotyped before and during the treatment course for CYP3A and ABCB1 (P-Glycoprotein) using midazolam and 99mTc-MIBI (methoxy-isobutyl-isonitrile) (43). Although at that place was a correlation between midazolam metabolic ratio and sunitinib pharmacokinetics, it was not sufficient to be useful for a clinical dosing strategy of sunitinib. This is in dissimilarity to an earlier publication, where the authors ended from a pocket-size sample size of 13 patients that midazolam and sunitinib exposure are highly correlated and therefore a large proportion of the observed interpatient variability of sunitinib exposure could be explained by CYP3A variability (45). Yet, looking at the data provided in the figures, variability of sunitinib (plus its metabolite) was just 2-fold and midazolam variability was two.v-fold (45). Analysing the drug interaction data available for sunitinib, information technology must be concluded that sunitinib clearance mechanisms are non yet well understood. The strong CYP3A inhibitor ketoconazole results in an AUCR of ane.51 (35), grapefruit juice every bit a selective CYP3A inhibitor in the GI-tract showed an AUCR of ane.11 (46), induction of CYP3A by rifampicin acquired an AUCR of 0.54 (35). In terms of clearance, ketoconazole volition reduce sunitinib clearance from 600 to 400 mL/min and rifampicin volition increment clearance to 1,200 mL/min. Knowing that ketoconazole is able to reduce at least 80% of CYP3A activity (47), there is even so sufficient clearance available to eliminate sunitinib probably mediated by other still unknown mechanisms.
Hereafter perspectives
For near of the TKIs a stock-still dose is used for every patient. Almost no dose recommendations or rules for dose adaptations are given. With respect to drug-drug interactions, TKIs are non listed as drugs or drug classes being prone to life-threatening drug-drug interactions (48). Nosotros need to develop indices that volition help us to determine when and how to alter the recommended dosing scheme of TKIs. TDM can of course exist very helpful in adjusting the dose to achieve the required concentrations. At that place have been methods developed, where numerous TKIs tin can be quantified with the aforementioned belittling method, thereby facilitating an like shooting fish in a barrel and reliable manner to ensure timely drug quantifications (49). Manifestly, but a few centres accept established TDM for TKI dose optimisation (49). Another possibility might be the use of phenotyping to assess the activity of the principle enzyme(southward) involved in metabolism. In club to use phenotyping, it is a prerequisite to accept data on the fraction captivated, the clearance mechanisms involved in elimination, and their relative contribution to the overall clearance. Information technology should be an absolute requirement for drug companies to provide these information to the regulatory agencies. With this knowledge, it volition exist possible in future to use a probe drug cocktail to assess the private enzyme and transporter activities at any time necessary. If microdoses are used, the patient is not burdened with drugs and would not endure from side furnishings of the probe drugs. This has already been established for midazolam as a probe drug for CYP3A activity (4,l,51); even with a limited sampling strategy involved (52).
Lesson to exist learned
If drugs show a human relationship betwixt plasma exposure and drug efficacy and/or toxicity clearance pathways must be examined to evaluate the relative contribution of CYP3A to the overall clearance. If CYP3A accounts for less than 25% of total clearance, there is a loftier probability that no dose adaptation due to CYP3A mediated drug interactions volition be necessary. If CYP3A contributes to 50% or more to overall clearance, phenotyping might exist applicative in time to come to adjust the dose to the individual activity and be so validated by TDM.
Acknowledgments
Funding: None.
Provenance and Peer Review: This article was commissioned past the Guest Editors (Michael Sorich and Andrew Rowland) for the series "Precision dosing of targeted anticancer drugs" published in Translational Cancer Inquiry. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure grade (available at http://dx.doi.org/10.21037/tcr.2017.09.x). The serial "Precision dosing of targeted anticancer drugs" was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accurateness or integrity of whatsoever part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND four.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original piece of work is properly cited (including links to both the formal publication through the relevant DOI and the license). Encounter: https://creativecommons.org/licenses/past-nc-nd/4.0/.
References
- Chabner B, Barnes J, Neal J, et al. Targeted therapies: tyrosine kinase inhibitors, monoclonal antibodies, and Cytokines. In: Brunton LL, Chabner BA, Knollmann BC, editors. Goodman & Gilman'southward The Pharmacological Basis of Therapeutics, Twelfth Edition. NY: McGraw-Colina Education, 2011;1731-69.
- Zhou SF. Drugs behave equally substrates, inhibitors and inducers of homo cytochrome P450 3A4. Curr Drug Metab 2008;ix:310-22. [Crossref] [PubMed]
- Backman JT, Kivisto KT, Olkkola KT, et al. The area under the plasma concentration-time curve for oral midazolam is 400-fold larger during treatment with itraconazole than with rifampicin. Eur J Clin Pharmacol 1998;54:53-8. [Crossref] [PubMed]
- Hohmann Northward, Haefeli We, Mikus One thousand. CYP3A activeness: towards dose adaptation to the private. Expert Opin Drug Metab Toxicol 2016;12:479-97. [Crossref] [PubMed]
- US-Nutrient and Drug Administration. Guidance for industry: drug interaction studies—study blueprint, data assay, implications for dosing, and labeling recommendations (typhoon guidance). 2012. Available online: https://www.fda.gov/downloads/drugs/guidances/ucm292362.pdf
- European Medicines Agency. Guideline on the investigation of drug interactions: final (CPMP/EWP/560/95/Rev. 1 corr. two). 2012. Bachelor online: http://www.ema.europa.european union/docs/en_GB/document_library/Scientific_guideline/2012/07/WC500129606.pdf
- Rowland A, van Dyk 1000, Mangoni AA, et al. Kinase inhibitor pharmacokinetics: comprehensive summary and roadmap for addressing inter-private variability in exposure. Expert Opin Drug Metab Toxicol 2017;xiii:31-49. [Crossref] [PubMed]
- van Leeuwen RW, van Gelder T, Mathijssen RH, et al. Drug-drug interactions with tyrosine-kinase inhibitors: a clinical perspective. Lancet Oncol 2014;fifteen:e315-26. [Crossref] [PubMed]
- European Medicines Agency. Glivec: EPAR - Scientific Discussion. 2004. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000406/WC500022203.pdf
- European Medicines Agency. EMA/CHMP/726072/2014 - CHMP assessment report Vargatef. 2014. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002569/WC500179972.pdf
- European Medicines Agency. EMEA/302222/2008 - ASSESSMENT REPORT FOR TYVERB. 2008. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/000795/WC500044960.pdf
- European Medicines Agency. EMA/491185/2013 - CHMP assessment report Giotrif. 2013. Bachelor online: http://www.ema.europa.european union/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002280/WC500152394.pdf
- European Medicines Agency. EMA/10821/2017 - CHMP assessment report Alecensa. 2016. Bachelor online: http://world wide web.ema.europa.european union/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/004164/WC500225709.pdf
- European Medicines Agency. EMA/CHMP/453325/2012 - CHMP cess report Inlyta. 2012. Bachelor online: http://world wide web.ema.europa.european union/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human being/002406/WC500132190.pdf
- European Medicines Bureau. EMA/70979/2013 - CHMP assessment report Bosulif. 2013. Bachelor online: http://www.ema.europa.european union/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human being/002373/WC500141745.pdf
- European Medicines Bureau. EMA/664123/2016 - CHMP cess report CABOMETYX. 2016. Bachelor online: http://world wide web.ema.europa.european union/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/004163/WC500214070.pdf
- European Medicines Agency. EMA/170114/2015 - CHMP assessment report Zykadia. 2015. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human being/003819/WC500187506.pdf
- European Medicines Agency. EMA/685908/2015 - CHMP assessment study Cotellic. 2015. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/003960/WC500198565.pdf
- European Medicines Bureau. EMA/CHMP/497137/2012 - CHMP assessment written report XALKORI. 2012. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002489/WC500134761.pdf
- European Medicines Agency. EMA/CHMP/242419/2013/corr 1 - CHMP cess report Tafinlar. 2013. Available online: http://world wide web.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002604/WC500149673.pdf
- European Medicines Agency. Sprycel: EPAR - Scientific Word. 2006. Bachelor online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000709/WC500056995.pdf
- European Medicines Agency. Tarceva: EPAR - Scientific Discussion. 2005. Bachelor online: http://world wide web.ema.europa.european union/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000618/WC500033991.pdf
- European Medicines Bureau. EMEA/CHMP/563746/2008 - CHMP assessment report Iressa. 2009. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/001016/WC500036361.pdf
- European Medicines Agency. EMA/CHMP/645137/2014 - CHMP assessment report Imbruvica. 2014. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human being/003791/WC500177777.pdf
- European Medicines Agency. EMEA/CHMP/324336/2014 - CHMP cess report Zydelig. 2014. Available online: http://world wide web.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/homo/003843/WC500175379.pdf
- European Medicines Agency. EMA/250082/2015 - CHMP assessment written report Lenvima. 2015. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/003727/WC500188676.pdf
- European Medicines Agency. Tasigna: EPAR - Scientific Discussion. 2007. Bachelor online: http://world wide web.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/homo/000798/WC500034398.pdf
- European Medicines Agency. EMA/CHMP/15445/20165 - CHMP assessment report TAGRISSO. 2015. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/homo/004124/WC500202024.pdf
- European Medicines Bureau. EMA/652627/2016 - CHMP cess report IBRANCE. 2016. Bachelor online: http://world wide web.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/003853/WC500217198.pdf
- European Medicines Agency. EMA/CHMP/248579/2010 - CHMP assessment report Votrient. 2010. Bachelor online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/001141/WC500094275.pdf
- European Medicines Agency. EMA/CHMP/220290/2013 - CHMP assessment written report Iclusig. 2013. Available online: http://www.ema.europa.european union/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human being/002695/WC500145648.pdf
- European Medicines Agency. EMA/CHMP/403683/2013 - CHMP assessment written report Stivarga. 2013. Bachelor online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002573/WC500149166.pdf
- European Medicines Agency. EMA/465846/2012 - CHMP assessment written report Jakavi. 2012. Bachelor online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002464/WC500133226.pdf
- European Medicines Agency. Nexavar: EPAR - Scientific Word. 2007. Available online: http://world wide web.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/man/000690/WC500027707.pdf
- European Medicines Agency. Sutent: EPAR - Scientific Discussion. 2007. Bachelor online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000687/WC500057733.pdf
- European Medicines Agency. EMA/CHMP/853224/2016 - CHMP assessment written report Xeljanz. 2017. Available online: http://www.ema.europa.european union/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/004214/WC500224913.pdf
- European Medicines Agency. EMA/CHMP/258608/2014 - CHMP cess written report Mekinist. 2014. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/man/002643/WC500169708.pdf
- European Medicines Bureau. EMA/128076/2012 - CHMP assessment report Caprelsa. 2012. Available online: http://world wide web.ema.europa.european union/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002315/WC500123603.pdf
- European Medicines Bureau. EMA/200986/2012 - CHMP assessment written report Zelboraf. 2012. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002409/WC500124400.pdf
- Speed B, Bu HZ, Puddle WF, et al. Pharmacokinetics, distribution, and metabolism of [14C]sunitinib in rats, monkeys, and humans. Drug Metab Dispos 2012;40:539-55. [Crossref] [PubMed]
- Houk Be, Bello CL, Poland B, et al. Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: results of a pharmacokinetic/pharmacodynamic meta-analysis. Cancer Chemother Pharmacol 2010;66:357-71. [Crossref] [PubMed]
- Houk BE, Bello CL, Kang D, et al. A population pharmacokinetic meta-analysis of sunitinib malate (SU11248) and its primary metabolite (SU12662) in healthy volunteers and oncology patients. Clin Cancer Res 2009;xv:2497-506. [Crossref] [PubMed]
- Kloth JS, Binkhorst L, de Wit Every bit, et al. Relationship Between Sunitinib Pharmacokinetics and Administration Time: Preclinical and Clinical Evidence. Clin Pharmacokinet 2015;54:851-8. [Crossref] [PubMed]
- US-Nutrient and Drug Administration. SUTENT: Prescribing information. 2015. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/characterization/2015/021938s028s029lbl.pdf
- de Wit D, Gelderblom H, Sparreboom A, et al. Midazolam as a phenotyping probe to predict sunitinib exposure in patients with cancer. Cancer Chemother Pharmacol 2014;73:87-96. [Crossref] [PubMed]
- van Erp NP, Bakery SD, Zandvliet AS, et al. Marginal increment of sunitinib exposure past grapefruit juice. Cancer Chemother Pharmacol 2011;67:695-703. [Crossref] [PubMed]
- Fuchs I, Hafner-Blumenstiel V, Markert C, et al. Consequence of the CYP3A inhibitor ketoconazole on the PXR-mediated induction of CYP3A activity. Eur J Clin Pharmacol 2013;69:507-xiii. [Crossref] [PubMed]
- Solar day RO, Snowden L, McLachlan AJ. Life-threatening drug interactions: what the physician needs to know. Intern Med J 2017;47:501-12. [Crossref] [PubMed]
- van Dyk Thousand, Miners JO, Kichenadasse G, et al. A novel arroyo for the simultaneous quantification of 18 pocket-sized molecule kinase inhibitors in human plasma: a platform for optimised KI dosing. J Chromatogr B Analyt Technol Biomed Life Sci 2016;1033-1034:17-26. [Crossref] [PubMed]
- Halama B, Hohmann Northward, Burhenne J, et al. A nanogram dose of the CYP3A probe substrate midazolam to evaluate drug interactions. Clin Pharmacol Ther 2013;93:564-71. [Crossref] [PubMed]
- Hohmann N, Halama B, Siller N, et al. Response to "can CYP3A activity be evaluated for drug interaction using a nanogram dose of probe drug?": evaluation of CYP3A activity with microdoses of midazolam. Clin Pharmacol Ther 2014;95:490-1. [Crossref] [PubMed]
- Katzenmaier S, Markert C, Riedel KD, et al. Determining the fourth dimension course of CYP3A inhibition by potent reversible and irreversible CYP3A inhibitors using a limited sampling strategy. Clin Pharmacol Ther 2011;90:666-73. [Crossref] [PubMed]
Cite this article as: Mikus G, Foerster KI. Function of CYP3A4 in kinase inhibitor metabolism and assessment of CYP3A4 activity. Transl Cancer Res 2017;vi(Suppl 10):S1592-S1599. doi: 10.21037/tcr.2017.09.x
What Are Cyp3a4 Inhibitors Used For,
Source: https://tcr.amegroups.com/article/view/16370/html
Posted by: harkinshicle1975.blogspot.com


0 Response to "What Are Cyp3a4 Inhibitors Used For"
Post a Comment