Reactant In A Chemical Reaction
In chemistry, a reaction mechanism is the stride by step sequence of elementary reactions by which overall chemical change occurs.[1]
A chemic machinery is a theoretical conjecture that tries to describe in detail what takes identify at each stage of an overall chemic reaction. The detailed steps of a reaction are not observable in near cases. The conjectured mechanism is called because it is thermodynamically viable, and has experimental back up in isolated intermediates (see adjacent section) or other quantitative and qualitative characteristics of the reaction. It besides describes each reactive intermediate, activated complex, and transition state, and which bonds are broken (and in what society), and which bonds are formed (and in what order). A complete mechanism must as well explain the reason for the reactants and catalyst used, the stereochemistry observed in reactants and products, all products formed and the amount of each.

The electron or pointer pushing method is oft used in illustrating a reaction mechanism; for example, see the illustration of the mechanism for benzoin condensation in the following examples section.
A reaction mechanism must also account for the order in which molecules react. Frequently what appears to exist a single-footstep conversion is in fact a multistep reaction.
Reaction intermediates [edit]
Reaction intermediates are chemical species, often unstable and brusk-lived (nonetheless sometimes can be isolated), which are not reactants or products of the overall chemical reaction, merely are temporary products and/or reactants in the mechanism's reaction steps. Reaction intermediates are often costless radicals or ions.
The kinetics (relative rates of the reaction steps and the rate equation for the overall reaction) are explained in terms of the energy needed for the conversion of the reactants to the proposed transition states (molecular states that corresponds to maxima on the reaction coordinates, and to saddle points on the potential energy surface for the reaction).
Chemical kinetics [edit]
Information about the mechanism of a reaction is often provided by the use of chemic kinetics to decide the rate equation and the reaction lodge in each reactant.[2]
Consider the post-obit reaction for example:
- CO + NOii → CO2 + NO
In this case, experiments have determined that this reaction takes place according to the charge per unit police . This form suggests that the rate-determining stride is a reaction between two molecules of NOii. A possible mechanism for the overall reaction that explains the rate law is:
- 2 NOii → NOiii + NO (tiresome)
- NO3 + CO → NO2 + CO2 (fast)
Each step is called an uncomplicated stride, and each has its ain rate law and molecularity. The unproblematic steps should add up to the original reaction. (Meaning, if we were to abolish out all the molecules that appear on both sides of the reaction, nosotros would be left with the original reaction.)
When determining the overall rate constabulary for a reaction, the slowest pace is the pace that determines the reaction rate. Because the first step (in the above reaction) is the slowest stride, it is the rate-determining step. Because information technology involves the standoff of two NO2 molecules, it is a bimolecular reaction with a charge per unit which obeys the rate law .
Other reactions may have mechanisms of several consecutive steps. In organic chemistry, the reaction mechanism for the benzoin condensation, put frontwards in 1903 by A. J. Lapworth, was one of the first proposed reaction mechanisms.
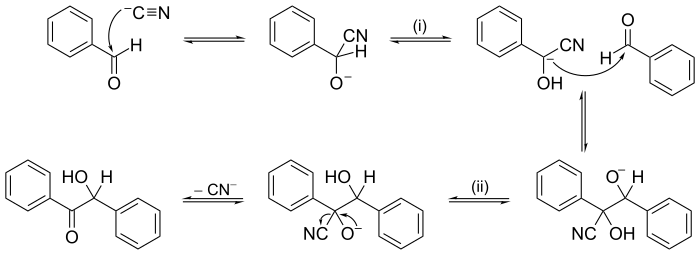
Benzoin condensation reaction mechanism. Cyanide ion (CN−) acts as a goad here, entering at the beginning stride and leaving in the last step. Proton (H+) transfers occur at (i) and (ii). The arrow pushing method is used in some of the steps to show where electron pairs go.
A chain reaction is an example of a circuitous mechanism, in which the propagation steps form a closed cycle. In a chain reaction, the intermediate produced in one footstep generates an intermediate in another step. Intermediates are called concatenation carriers. Sometimes, the chain carriers are radicals, they can be ions as well. In nuclear fission they are neutrons.
Chain reactions have several steps, which may include:[3]
- Chain initiation: this can be past thermolysis (heating the molecules) or photolysis (absorption of light) leading to the breakage of a bond.
- Propagation: a chain carrier makes another carrier.
- Branching: one carrier makes more than one carrier.
- Retardation: a chain carrier may react with a product reducing the rate of germination of the production. It makes another chain carrier, but the product concentration is reduced.
- Chain termination: radicals combine and the chain carriers are lost.
- Inhibition: chain carriers are removed by processes other than termination, such equally by forming radicals.
Fifty-fifty though all these steps can appear in one chain reaction, the minimum necessary ones are: Initiation, propagation and termination.
An instance of a elementary chain reaction is the thermal decomposition of acetaldehyde (CH3CHO) to methane (CHfour) and carbon monoxide (CO). The experimental reaction order is 3/2,[four] which can be explained by a Rice-Herzfeld machinery.[five]
This reaction mechanism for acetaldehyde has 4 steps with rate equations for each step :
- Initiation : CH3CHO → •CH3 + •CHO (Rate=kone [CHiiiCHO])
- Propagation: CHiiiCHO + •CHiii → CH4 + CH3CO• (Rate=yardtwo [CH3CHO][•CH3])
- Propagation: CH3CO• → •CHthree + CO (Rate=k3 [CHiiiCO•])
- Termination: •CHthree + •CH3 → CH3CHiii (Charge per unit=k4 [•CHthree]two)
For the overall reaction, the rates of alter of the concentration of the intermediates •CH3 and CH3CO• are zero, according to the steady-land approximation, which is used to account for the charge per unit laws of concatenation reactions.[6]
d[•CHiii]/dt = k1[CH3CHO] – k2[•CH3][CH3CHO] + k3[CH3CO•] - 2kiv[•CHiii]two = 0
and d[CHiiiCO•]/dt = mii[•CH3][CHiiiCHO] – one thousand3[CH3CO•] = 0
The sum of these two equations is k1[CH3CHO] – 2 thoufour[•CH3]two = 0. This may be solved to observe the steady-state concentration of •CH3 radicals as [•CH3] = (gone / 2k4)ane/ii [CHthreeCHO]1/two.
Information technology follows that the rate of germination of CHfour is d[CHiv]/dt = kii[•CH3][CH3CHO] = kii (m1 / 2k4)1/two [CH3CHO]3/2
Thus the mechanism explains the observed charge per unit expression, for the master products CHfour and CO. The exact rate law may be even more complicated, there are also minor products such as acetone (CHthreeCOCHthree) and propanal (CH3CHiiCHO).
Other experimental methods to determine mechanism [edit]
Many experiments that suggest the possible sequence of steps in a reaction mechanism have been designed, including:
- measurement of the outcome of temperature (Arrhenius equation) to make up one's mind the activation energy[7]
- spectroscopic ascertainment of reaction intermediates
- determination of the stereochemistry of products, for example in nucleophilic substitution reactions[viii]
- measurement of the result of isotopic commutation on the reaction rate[9]
- for reactions in solution, measurement of the effect of pressure on the reaction rate to make up one's mind the volume change on formation of the activated complex[10] [11]
- for reactions of ions in solution, measurement of the effect of ionic forcefulness on the reaction rate[12] [13]
- direct observation of the activated complex by pump-probe spectroscopy[xiv]
- infrared chemiluminescence to observe vibrational excitation in the products[15] [sixteen]
- electrospray ionization mass spectrometry.[17]
- crossover experiments.[18]
Theoretical modeling [edit]
A correct reaction machinery is an important office of accurate predictive modeling. For many combustion and plasma systems, detailed mechanisms are not available or require development.
Even when information is available, identifying and assembling the relevant data from a diversity of sources, reconciling discrepant values and extrapolating to dissimilar conditions can be a difficult procedure without expert help. Rate constants or thermochemical information are ofttimes not available in the literature, and then computational chemistry techniques or group additivity methods must be used to obtain the required parameters.
Computational chemistry methods can also be used to calculate potential energy surfaces for reactions and determine likely mechanisms.[19]
Molecularity [edit]
Molecularity in chemical science is the number of colliding molecular entities that are involved in a unmarried reaction pace.
- A reaction pace involving one molecular entity is chosen unimolecular.
- A reaction step involving two molecular entities is called bimolecular.
- A reaction footstep involving three molecular entities is chosen trimolecular or termolecular.
In general, reaction steps involving more than than three molecular entities practice non occur, because is statistically improbable in terms of Maxwell distribution to notice such transition land.
See too [edit]
- Organic reactions by mechanism
- Nucleophilic acyl substitution
- Neighbouring group participation
- Finkelstein reaction
- Lindemann mechanism
- Electrochemical reaction machinery
- Nucleophilic abstraction
References [edit]
- ^ March, Jerry (1985), Avant-garde Organic Chemistry: Reactions, Mechanisms, and Structure (3rd ed.), New York: Wiley, ISBN0-471-85472-7
- ^ Espenson, James H. Chemical Kinetics and Reaction Mechanisms (2d ed., McGraw-Hill, 2002) chap.six, Deduction of Reaction Mechanisms ISBN 0-07-288362-6
- ^ Bäckström, Hans L. J. (ane June 1927). "The chain-reaction theory of negative catalysis". Journal of the American Chemical Club. 49 (6): 1460–1472. doi:10.1021/ja01405a011. Retrieved 20 January 2021.
- ^ Laidler K.J. and Meiser J.H., Physical Chemistry (Benjamin/Cummings 1982) p.416-417 ISBN 0-8053-5682-7
- ^ Atkins and de Paula p.830-1
- ^ Atkins P and de Paula J, Physical Chemical science (8th ed., West.H. Freeman 2006) p.812 ISBN 0-7167-8759-eight
- ^ Espenson p.156-160
- ^ Morrison R.T. and Boyd R.North. Organic Chemical science (4th ed., Allyn and Bacon 1983) p.216-9 and p.228-231, ISBN 0-205-05838-viii
- ^ Atkins P and de Paula J, Physical Chemical science (8th ed., W.H. Freeman 2006) p.816-8 ISBN 0-7167-8759-eight
- ^ Moore J.West. and Pearson R.G. Kinetics and Machinery (third ed., John Wiley 1981) p.276-8 ISBN 0-471-03558-0
- ^ Laidler 1000.J. and Meiser J.H., Physical Chemistry (Benjamin/Cummings 1982) p.389-392 ISBN 0-8053-5682-7
- ^ Atkins and de Paula p.884-five
- ^ Laidler and Meiser p.388-9
- ^ Atkins and de Paula p.892-3
- ^ Atkins and de Paula p.886
- ^ Laidler and Meiser p.396-7
- ^ Investigation of chemical reactions in solution using API-MS Leonardo Silva Santos, Larissa Knaack, Jurgen O. Metzger Int. J. Mass Spectrom.; 2005; 246 pp 84 - 104; (Review) doi:ten.1016/j.ijms.2005.08.016
- ^ Espenson p.112
- ^ Atkins and de Paula p.887-891
L.Thousand.WADE,ORGANIC CHEMISTRY 7th ED,2010
External links [edit]
- Reaction mechanisms for combustion of hydrocarbons
Reactant In A Chemical Reaction,
Source: https://en.wikipedia.org/wiki/Reaction_mechanism
Posted by: harkinshicle1975.blogspot.com

![r=k[NO_{2}]^{2}](https://wikimedia.org/api/rest_v1/media/math/render/svg/7c34576960b3342cceed6c0b51b1764beb2dfcf4)

![{\displaystyle r=k[NO_{2}(t)]^{2}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/581dd3ba310fe8d53ab10b6a2ed319f4d67ed40a)

0 Response to "Reactant In A Chemical Reaction"
Post a Comment